Which Of The Following Describes Why Cytokinesis Is Different In Plant And Animal Cells?
The jail cell bicycle culminates in the sectionalization of the cytoplasm by cytokinesis. In a typical jail cell, cytokinesis accompanies every mitosis, although some cells, such as Drosophila embryos (discussed afterwards) and vertebrate osteoclasts (discussed in Chapter 22), undergo mitosis without cytokinesis and become multinucleate. Cytokinesis begins in anaphase and ends in telophase, reaching completion equally the next interphase begins.
The offset visible change of cytokinesis in an animal cell is the sudden appearance of a crease, or cleavage furrow, on the cell surface. The furrow rapidly deepens and spreads around the prison cell until information technology completely divides the prison cell in two. In creature cells and many unicellular eucaryotes, the structure that accomplishes cytokinesis is the contractile ring—a dynamic assembly composed of actin filaments, myosin II filaments, and many structural and regulatory proteins. The ring assembles just beneath the plasma membrane and contracts to constrict the cell into two (see Figure 18-4). At the aforementioned fourth dimension, new membrane is inserted into the plasma membrane adjacent to the contractile band by the fusion of intracellular vesicles. This addition of membrane is required to compensate for the increase in surface area that accompanies cytoplasmic division. Thus, cytokinesis can be considered to occur in 4 stages—initiation, contraction, membrane insertion, and completion.
The primal problem for a prison cell undergoing cytokinesis is to ensure that it occurs at the right time and in the right identify. Cytokinesis must non occur besides early in M-phase, or information technology volition disrupt the path of the separating chromosomes. It must also occur at the right place to separate the 2 segregating sets of chromosomes properly so that each daughter jail cell receives a complete set.
The Microtubules of the Mitotic Spindle Determine the Plane of Animate being Cell Division
The mitotic spindle in animate being cells not only separates the daughter chromosomes, it besides specifies the location of the contractile ring, and thereby the plane of cell division. The contractile band invariably forms in the plane of the metaphase plate, at right angles to the long axis of the mitotic spindle, thereby ensuring that division occurs between the two sets of separated chromosomes. The part of the spindle that specifies the division plane varies depending on the cell blazon: in some cells, it is the astral microtubules; in others, it is the overlapping antiparallel microtubules in the cardinal spindle.
The relationship between the spindle microtubules and the placement of the contractile ring has been studied by manipulating fertilized eggs of marine invertebrates. Later fertilization, these embryos undergo a series of rapid cleavage divisions, without intervening periods of growth. In this way, the original egg is progressively divided up into smaller and smaller cells. During cytokinesis, the cleavage furrow appears all of a sudden on the surface of the cell and deepens rapidly (Effigy eighteen-30). Because the cytoplasm is clear, the spindle tin can be observed in real time through a microscope. If the spindle is tugged into a new position with a fine glass needle in early on anaphase, the incipient cleavage furrow disappears, and a new one develops in accord with the new spindle site.

Figure 18-30
Cleavage in a fertilized frog egg. In these scanning electron micrographs, the cleavage furrow is specially obvious and well defined, as the cell is unusually large. The furrowing of the cell membrane is caused by the activity of the contractile ring (more...)
How does the mitotic spindle command the plane of sectionalisation? Ingenious experiments in big embryonic cells demonstrate that a cleavage furrow forms midway betwixt the asters originating from the two centrosomes, fifty-fifty when the two centrosomes are non connected to each other by a mitotic spindle (Figure 18-31). Thus, in these cells, the microtubule asters—not the chromosomes or other parts of the spindle—betoken to the cell cortex to specify where the contractile band should assemble. In other cells, the central spindle, rather than the astral microtubules, is plain responsible for this specification. In either case, it has been speculated that the overlapping microtubules may provide tracks for motor proteins to deliver contractile ring regulators, and peradventure new membrane, to the appropriate region of the dividing cell. But, in fact, the molecular mechanism by which the spindle positions the cleavage furrow remains a mystery.

Figure xviii-31
An experiment demonstrating the influence of the position of microtubule asters on the subsequent plane of cleavage in a large egg jail cell. If the mitotic spindle is mechanically pushed to one side of the jail cell with a glass bead, the membrane furrowing is (more...)
In some cells, the site of ring assembly is chosen before mitosis, according to a landmark placed in the cortex during a previous cell cycle. In budding yeasts, for example, a ring of proteins called septins assembles earlier mitosis, adjacent to a bud scar left on the cell surface every bit the mother and daughter cells separated in the previous division. The septins are thought to form a scaffold onto which other components of the contractile ring, including myosin II, assemble. Every bit nosotros discuss later, in plant cells, an organized ring of microtubules and actin filaments assembles simply before mitosis and marks the site where the cell wall will assemble and separate the cell in ii.
Some Cells Reposition Their Spindle to Divide Asymmetrically
Most cells split symmetrically. In nigh animal cells, for case, the contractile ring forms around the equator of the parent prison cell, so that the two daughter cells produced are of equal size and have similar properties. This symmetry results from the placement of the mitotic spindle, which in most cases tends to center itself in the cytoplasm. The centering process depends both on astral microtubules and on motor proteins that either button or pull on the astral microtubules to centre the spindle.
There are many instances in development, even so, when cells divide asymmetrically to produce two cells that differ in size, in the cytoplasmic contents they inherit, or in both. Usually, the two daughter cells are destined to develop along different pathways. To create girl cells with different fates, the female parent cell must first segregate some components (chosen fate determinants) to 1 side of the jail cell and so position the plane of partition and so that the appropriate daughter cell inherits these components (Figure 18-32). To position the plane of sectionalization asymmetrically, the spindle has to be moved in a controlled way within the dividing cell. It seems likely that such spindle movements are directed past changes in local regions of the prison cell cortex and that motor proteins localiazed there pull one of the spindle poles, via its astral microtubules, to the appropriate region (Figure 18-33). Some of the proteins required for such asymmetrical divisions have been identified through genetic analyses in C. elegans and Drosophila (discussed in Chapter 21), and some of these seem to have a similar role in vertebrates.

Figure 18-32
An asymmetric prison cell division segregating cytoplasmic components to only i girl jail cell. These low-cal micrographs illustrate the controlled asymmetric segregation of specific cytoplasmic components to one daughter prison cell during the first division of a fertilized (more...)

Figure 18-33
Spindle rotation. (A) A possible mechanism underlying the controlled rotation of a mitotic spindle. The red bar represents a specialized region of cell cortex toward which 1 spindle pole is pulled past its astral microtubules. (B) Fluorescence micrographs (more...)
Asymmetric division is specially of import in plant cells. Every bit these cells cannot move after division, the option of division planes is crucial for decision-making tissue morphology. Nosotros discuss later how the plane of division is adamant in these cells.
Actin and Myosin Two in the Contractile Ring Generate the Force for Cytokinesis
Equally the astral microtubules in anaphase become longer lived and less dynamic in response to the loss of M-Cdk activity, the contractile ring begins to get together beneath the plasma membrane. Much of the preparation for cytokinesis, however, happens before in mitosis, before the division of the cytoplasm really begins. In interphase cells, actin and myosin filaments are assembled into a cortical network and, in some cells, besides into large cytoplasmic bundles called stress fibers (discussed in Chapter 16). As cells enter mitosis, these arrays disassemble; much of the actin is reorganized, and myosin II filaments are released. Every bit the chromatids separate in anaphase, myosin II begins to accumulate in the apace assembling contractile ring (Figure 18-34).

Figure 18-34
The contractile ring. (A) A drawing of the cleavage furrow in a dividing cell. (B) An electron micrograph of the ingrowing edge of a cleavage furrow of a dividing animal cell. (C) Fluorescence micrographs of a dividing slime mold amoeba stained for actin (more...)
In many cells, cytokinesis requires the activation of one or more members of the polo-like family of protein kinases. These kinases regulate the associates of both the mitotic spindle and the contractile band and are therefore idea to assist coordinate mitosis and cytokinesis, but information technology is uncertain how they do and so. The fully assembled contractile ring contains many proteins in improver to actin and myosin II. The overlapping arrays of actin filaments and bipolar myosin II filaments, however, generate the force that divides the cytoplasm in two. They are thought to contract past a mechanism that is biochemically similar to that used by shine muscle cells; in both cases, for example, the contraction begins when Ca2+-calmodulin activates myosin light-chain kinase to phosphorylate myosin Two. Once contraction has been stimulated, the ring develops a force large enough to bend a fine drinking glass needle that is inserted in the path of the constricting ring.
How the contractile band constricts is yet a mystery. It seems non to operate by a unproblematic "purse-string" mechanism, with actin and myosin II filaments sliding by each other as in skeletal muscle (see Figure 16-71). As the ring constricts, the ring maintains the same thickness in cross-section, suggesting that its total volume and the number of filaments it contains decrease steadily. Moreover, different in muscle, the actin filaments in the ring are highly dynamic, and their organization changes extensively during cytokinesis.
In addition to specifying the site of contractile ring assembly in early anaphase, in many cells, microtubules also work continuously during anaphase and telophase to stabilize the advancing cleavage furrow. Drugs that depolymerize microtubules, for example, cause the actin filaments in the contractile band to become less organized. Moreover, if a needle is used to tear microtubules away from the cell cortex, the contractile ring disassembles and the cleavage furrow regresses. It is not known how the microtubules stabilize the ring, although it has been shown that growing microtubules can actuate some members of the Rho family unit of small GTPases, which in turn stimulate actin polymerization (discussed in Chapter 16). 1 member of this family, Rho A, is required for cytokinesis.
The contractile ring is finally dispensed with altogether when cleavage ends, as the plasma membrane of the cleavage furrow narrows to form the midbody. The midbody persists as a tether between the two girl cells and contains the remains of the central spindle, which now consists of the 2 sets of antiparallel overlap microtubules packed tightly together within a dumbo matrix material (Figure eighteen-35). Remarkably, in some cells, earlier cytokinesis has been completed, the mother centriole from one or both daughter cells separates from its daughter centriole (see Figure eighteen-5c) and migrates into the midbody, where it lingers for minutes, before returning to its daughter prison cell. Only then exercise the two daughter cells separate to complete cytokinesis. What the centriole might do in the midbody to trigger the final steps of cytokinesis is not known. Afterwards the daughter cells separate completely, some of the components of the residual midbody oft remain on the inside of the plasma membrane of each cell, where they may serve as a marking on the cortex that helps to orient the spindle in the subsequent cell division.

Figure 18-35
The midbody. (A) A scanning electron micrograph of an beast prison cell in culture in the process of dividing; the midbody still joins the two girl cells. (B) A conventional electron micrograph of the midbody of a dividing animal cell. Cleavage is virtually (more...)
Membrane-enclosed Organelles Must Exist Distributed to Daughter Cells During Cytokinesis
The process of mitosis ensures that each daughter jail cell receives a total complement of chromosomes. But when a eucaryotic prison cell divides, each daughter cell must also inherit all of the other essential cell components, including the membrane-enclosed organelles. As discussed in Chapter 12, organelles similar mitochondria and chloroplasts cannot assemble spontaneously from their individual components; they tin can ascend only from the growth and sectionalization of the preexisting organelles. Similarly, cells cannot make a new endoplasmic reticulum (ER) unless some part of it is already present.
How, then, are the various membrane-enclosed organelles segregated when a prison cell divides? Organelles such as mitochondria and chloroplasts are usually nowadays in big enough numbers to exist safely inherited if, on average, their numbers roughly double one time each cycle. The ER in interphase cells is continuous with the nuclear membrane and is organized by the microtubule cytoskeleton. Upon entry into M phase, the reorganization of the microtubules releases the ER, which fragments as the nuclear envelope breaks downwardly. The Golgi appliance probably fragments as well, although in some cells information technology seems to redistribute transiently into the ER, only to re-emerge at telophase. Some of the organelle fragments associate with the spindle microtubules via motor proteins, thereby hitching a ride into the girl cells as the spindle elongates in anaphase.
Mitosis Can Occur Without Cytokinesis
Although nuclear division is usually followed by cytoplasmic division, there are exceptions. Some cells undergo multiple rounds of nuclear sectionalisation without intervening cytoplasmic division. In the early Drosophila embryo, for example, the first thirteen rounds of nuclear division occur without cytoplasmic sectionalisation, resulting in the formation of a single large cell containing 6000 nuclei, arranged in a monolayer near the surface (Figure 18-36). This organization greatly speeds upwards early development, as the cells do not have to take the time to go through all the steps of cytokinesis for each division. Later these rapid nuclear divisions, cells are created around each nucleus in one circular of coordinated cytokinesis chosen cellularization. Contractile rings class at the cell surface, and the plasma membrane extends inward and pinches off to enclose each nucleus.

Figure 18-36
Mitosis without cytokinesis in the Drosophila embryo. (A) The start 13 nuclear divisions occur synchronously and without cytoplasmic sectionalisation to create a big syncytium. Near of the nuclei and then migrate to the cortex, and the plasma membrane extends (more...)
Nuclear division without cytokinesis likewise occurs in some types of mammalian cells. Osteoclasts, trophoblasts, and some hepatocytes and heart musculus cells, for example, go multinucleated in this manner.
The Phragmoplast Guides Cytokinesis in Higher Plants
Nearly college-plant cells are enclosed by a semirigid cell wall, and their machinery of cytokinesis is dissimilar from that just described for animal cells. Rather than a contractile ring dividing the cytoplasm from the outside in, the cytoplasm of the plant cell is partitioned from the inside out by the structure of a new jail cell wall, called the cell plate, between the two girl nuclei (Figure xviii-37). The orientation of the prison cell plate determines the positions of the ii daughter cells relative to neighboring cells. It follows that altering the planes of cell division, together with enlargement of the cells by expansion or growth, leads to different cell and tissue shapes that help determine the form of the plant.

Effigy 18-37
Cytokinesis in a found cell in telophase. In this lite micrograph, the early cell plate (betwixt the 2 arrowheads) is forming in a aeroplane perpendicular to the plane of the page. The microtubules of the spindle are stained with gold-labeled antibodies (more...)
The mitotic spindle past itself is not sufficient to determine the exact position and orientation of the cell plate. The first visible sign that a higher-institute cell has become committed to dissever in a particular plane is seen in Gtwo, when the cortical array of microtubules disappears in preparation for mitosis. At this time, a circumferential band of microtubules and actin filaments forms a band effectually the entire prison cell just below the plasma membrane. Because this cytoskeletal array appears before prophase begins, it is called the preprophase ring. The band becomes thinner as the jail cell progresses to prophase, and information technology disappears completely before metaphase is reached. Withal, the division aeroplane has somehow been established: when the new cell plate forms later during cytokinesis, it grows outward to fuse with the parental wall precisely at the zone formerly occupied past the preprophase band. Even if the cell contents are displaced by centrifugation after the preprophase band has disappeared, the growing cell plate tends to notice its manner back to the airplane defined by the onetime preprophase band.
The assembly of the prison cell plate begins in late anaphase and is guided by a structure called the phragmoplast, which contains the remaining overlap microtubules of the mitotic spindle that interdigitate at their growing plus ends. This region of overlap is similar in structure to the fundamental spindle in animal cells in late anaphase. Small vesicles, largely derived from the Golgi apparatus and filled with polysaccharide and glycoproteins required for the synthesis of the new cell-wall matrix, are transported along the microtubules to the equator of the phragmoplast, apparently by the activeness of microtubule-dependent motor proteins. Here, the vesicles fuse to grade a disclike, membrane-enclosed structure called the early cell plate (see Figure eighteen-9G). The plate expands outward by further vesicle fusion until it reaches the plasma membrane and the original cell wall and divides the cell in two. Later, cellulose microfibrils are laid downward within the matrix of the cell plate to consummate the construction of the new jail cell wall (Figure xviii-38).

Figure xviii-38
The special features of cytokinesis in a higher found prison cell. The division airplane is established before M phase by a ring of microtubules and actin filaments (the preprophase band) at the cell cortex. At the outset of telophase, after the chromosomes (more than...)
The Elaborate M Phase of Higher Organisms Evolved Gradually from Procaryotic Fission Mechanisms
Procaryotic cells carve up by a procedure called binary fission. The unmarried, circular Dna molecule replicates and division occurs by the invagination of the plasma membrane and the laying down of new cell wall between the two chromosomes to produce two separate girl cells. In E. coli, before the chromosome replicates, the single origin of replication (oriC) is located at one pole of the rod-shaped bacterium. As soon equally oriC is replicated, 1 copy of the sequence is immediately translocated to the contrary pole of the jail cell, after which the residue of the chromosome is replicated. Similar the two spindle-pole asters in an animal cell, the bacterial daughter chromosomes at the prison cell poles somehow determine the location of the plane of cell partitioning, ensuring that fission takes place at the cell equator, then that each daughter cell inherits one chromosome (Figure 18-39). Although a number of genes and proteins involved take been identified, the mechanisms responsible for the active translocation of oriC and the inhibition of fission everywhere but at the equator remain unknown.
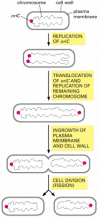
Figure 18-39
Cell sectionalisation in the bacterium E. coli. The single, circular chromosome contains an origin of replication called oriC. Before segmentation, the chromosome is polarized, and then that oriC is at i pole of the bacterium. As soon as the oriC sequence is copied, (more...)
Binary fission in procaryotes depends on filaments fabricated of the FtsZ protein. FtsZ is a cytoskeletal GTPase that is structurally related to tubulin and assembles into a ring at the equator of the jail cell (Figure eighteen-40A, and see Figure sixteen-17). The FtsZ filaments are essential for the recruitment of all the other prison cell partition proteins to the division site. Together, these proteins guide the inward growth of the cell wall and membrane, leading to the formation of a septum that divides the cell into two. Bacteria in which the ftsZ gene is inactivated past mutation cannot divide. A FtsZ-based mechanism is also used in the division of chloroplasts in institute cells (Figure eighteen-40B) and mitochondria in protists. In fungi and animal cells, another self-assembling GTPase called dynamin (discussed in Affiliate 13) has manifestly taken over the function of FtsZ in mitochondrial division.
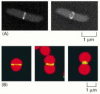
Figure 18-twoscore
The FtsZ protein. (A) Fluorescence micrographs showing the location of the FtsZ protein during binary fission in E. coli. The protein assembles into a ring at the center of the cell, where information technology helps orchestrate prison cell division. The bacteria here have been (more...)
With the development of the eucaryotes, the genome increased in complexity, and the chromosomes increased in both number and size. For these organisms, a more than elaborate mechanism for dividing the chromosomes between daughter cells was manifestly required. Clearly, the mitotic appliance could not have evolved all at in one case. In many primitive eucaryotes, such as the dinoflagellate Cryphthecodinium cohnii, mitosis depends on a membrane-attachment mechanism, in which the chromosomes have to bind to the inner nuclear membrane for segregation. The intermediate status of this large, single-celled alga is reflected in the composition of its chromosomes, which, like those of procaryotes, have relatively little associated protein. The nuclear membrane in C. cohnii remains intact throughout mitosis, and the spindle microtubules remain entirely outside the nucleus. Where these spindle microtubules press on the outside of the nuclear envelope, the envelope becomes indented in a serial of parallel channels (Figure 18-41). The chromosomes become attached to the inner membrane of the nuclear envelope reverse these channels, and chromosome segregation occurs on the inside of this channeled nuclear membrane. Thus, the extranuclear "spindle" is used to order the nuclear membrane and thereby define the airplane of sectionalisation. Kinetochores in this species seem to exist integrated into the nuclear membrane and may therefore take evolved from some membrane component.
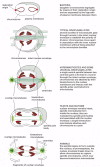
Effigy xviii-41
The use of different chromosome separation mechanisms past different organisms. Some of these may have been intermediate stages in the evolution of the mitotic spindle of college organisms. For all examples except bacteria, only the central nuclear region (more...)
Eucaryotic tubulin and procaryotic FtsZ clearly have a mutual evolutionary history. But, microtubules are important for chromosome segregation in even the nearly primitive eucaryotes, where they are also nowadays in flagellar axonemes (discussed in Chapter 16). Whether the flagellum or the spindle evolved commencement is unclear.
A somewhat more advanced, although withal extranuclear, spindle is seen in hypermastigotes, in which the nuclear envelope again remains intact throughout mitosis. These large protozoa from the guts of insects provide a particularly clear illustration of the independence of spindle elongation and the chromosome movements that separate the chromatids. The sis kinetochores get separated past the growth of the nuclear membrane (to which they are fastened) before becoming attached to the spindle. Only when the kinetochores are near the poles of the spindle do they learn the kinetochore microtubules needed to attach them to the spindle. Because the spindle microtubules remain separated from the chromosomes by the nuclear envelope, the kinetochore microtubules, which are formed outside the nucleus, must somehow adhere to the chromosomes through the nuclear membranes. After this attachment has occurred, the kinetochores are drawn poleward in a conventional manner (run into Figure 18-41).
Organisms that form spindles inside an intact nucleus may correspond a farther stage in the development of mitotic mechanisms. In both yeasts and diatoms, the spindle is attached to chromosomes by their kinetochores, and the chromosomes are segregated in a style loosely like to that described for animal cells—except that the entire procedure generally occurs within the confines of the nuclear envelope (come across Figure 18-41). Information technology is idea that the "open" mitosis of college organisms and the "airtight" mitosis of yeasts and diatoms evolved separately from a mutual ancestor resembling the modernistic hypermastigote spindle. At present, there is no disarming explanation for why college plants and animals accept evolved a mitotic apparatus that requires the controlled and reversible dissolution of the nuclear envelope.
Summary
Cell sectionalisation ends as the cytoplasm divides into two by the process of cytokinesis. Except for plants, cytokinesis in eucaryotic cells is mediated past a contractile band, which is composed of actin and myosin filaments and a variety of other proteins. By an unknown mechanism, the mitotic spindle determines when and where the contractile ring assembles and, thereby, when and where the prison cell divides. Most cells split up symmetrically to produce 2 cells of the same content and size. Some cells, however, specifically position their spindle to divide asymmetrically, producing two girl cells that differ in size, content, or both. Cytokinesis occurs by a special machinery in college-plant cells—in which the cytoplasm is partitioned past the structure of a new cell wall, the jail cell plate, inside the cell. The position of the cell plate is determined by the position of a preprophase ring of microtubules and actin filaments. The organization of mitosis in fungi and some protozoa differs from that in animals and plants, suggesting how the complex process of eucaryotic prison cell partition may have evolved.
Source: https://www.ncbi.nlm.nih.gov/books/NBK26831/
Posted by: deckertoomeng.blogspot.com

0 Response to "Which Of The Following Describes Why Cytokinesis Is Different In Plant And Animal Cells?"
Post a Comment